Hyperoxaluria Drug Market: Innovations in RNAi and Microbial Therapies Reshaping Rare Disease Treatment
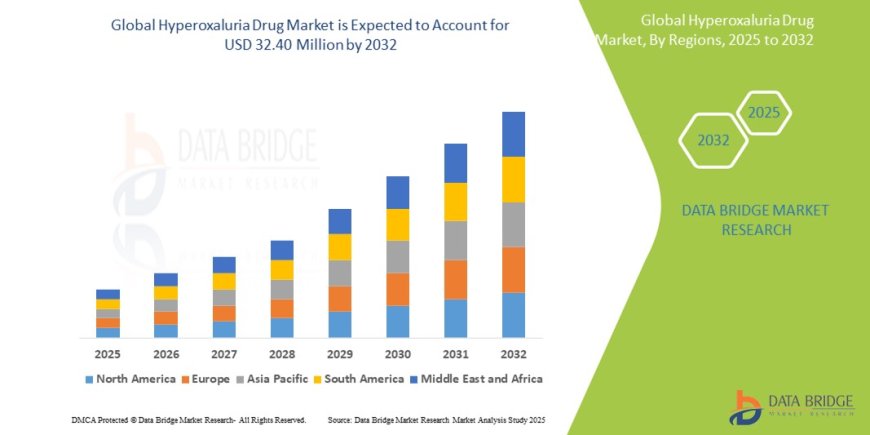
The global hyperoxaluria drug market size was valued at USD 19.43 million in 2024 and is expected to reach USD 32.40 million by 2032, at a CAGR of 6.6% during the forecast period
Introduction
Hyperoxaluria is a rare metabolic disorder characterized by the excessive accumulation of oxalate in the urine, which can lead to kidney stones, nephrocalcinosis, and eventually renal failure. It is broadly categorized into primary and secondary types, with Primary Hyperoxaluria Type 1 (PH1) being the most severe. In recent years, the hyperoxaluria drug market has experienced significant progress, thanks to advancements in RNA interference (RNAi) therapies, microbiome-based treatments, and novel small molecules. This market is expected to grow steadily due to improved diagnostics, increasing disease awareness, and the development of disease-modifying therapies.
Market Overview
The global hyperoxaluria drug market is in a transformative phase. The introduction of targeted therapies such as Lumasiran (Oxlumo), the first RNAi drug approved for PH1, has marked a major breakthrough. Market size is anticipated to expand at a robust CAGR due to growing diagnosis rates and the approval pipeline for additional treatment options.
Key Market Drivers
-
Innovative Therapies: RNAi-based drugs like Lumasiran and Nedosiran are changing the treatment paradigm by targeting the genetic cause of the disease.
-
Increased Diagnostic Capabilities: Improved genetic testing and early screening are enabling timely identification and management.
-
Rising Awareness and Advocacy: Support from rare disease organizations is helping boost research funding and patient access to emerging therapies.
-
Orphan Drug Designations: Regulatory incentives such as fast-track approvals, market exclusivity, and tax credits are attracting pharmaceutical investment.
Leading Therapies and Pipeline Candidates
-
Lumasiran (Oxlumo) – Approved by the FDA and EMA for PH1; it reduces oxalate production by silencing the HAO1 gene.
-
Nedosiran (Rivfloza) – Recently approved RNAi therapy targeting LDHA to reduce oxalate production in PH1 patients.
-
CHK-336 – A novel oral LDHA inhibitor currently in clinical trials.
-
Oxabact – A microbiome therapy using Oxalobacter formigenes, under development to degrade oxalate in the gut.
Regional Insights
-
North America dominates the market, led by early drug approvals, strong R&D infrastructure, and favorable reimbursement policies.
-
Europe is witnessing growing clinical trial activity and drug adoption, especially in countries with robust rare disease registries.
-
Asia-Pacific shows potential for growth due to improving healthcare access and genetic testing facilities, though market penetration remains in early stages.
Challenges in the Market
-
High Treatment Costs: Novel therapies, particularly RNAi drugs, come with significant costs that may limit accessibility.
-
Small Patient Population: Hyperoxaluria's rarity makes clinical trials and commercialization challenging.
-
Regulatory Hurdles: Navigating global regulatory pathways for rare disease treatments can delay product launches.
Future Outlook
The hyperoxaluria drug market is expected to grow substantially over the next decade, driven by the expanding adoption of RNAi therapies, the emergence of gene-editing approaches, and continued investment in microbiome-based solutions. The pipeline is rich with candidates that promise more targeted and patient-friendly treatment options. With supportive regulatory frameworks and growing collaboration between industry and advocacy groups, the future of hyperoxaluria management looks promising.
Conclusion
The hyperoxaluria drug market is on the cusp of a significant evolution. From symptomatic management to disease-modifying treatments, innovation is accelerating progress for patients with this debilitating disorder. As awareness and research continue to grow, the market is poised to deliver safer, more effective, and accessible therapies that offer renewed hope to patients worldwide.
Get More Details:
https://www.databridgemarketresearch.com/reports/global-hyperoxaluria-drug-market
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0






































